mrsamct
Registred
Joined: 06 Aug 2011
Posts: 207
|
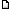 Posted: Thu Aug 11, 2011 4:21 am Post subject: Properties of Silicon Dioxide Posted: Thu Aug 11, 2011 4:21 am Post subject: Properties of Silicon Dioxide |
 |
|
buy website
warner bros dreamworld car hire
We all remember visiting sandy beaches in our childhood. What did we do then? Well, we used to carve castles in the sand and beam at our creations! But have you ever wondered what the sand might look like at the molecular level? What might happen, say, if we burnt the sand or dissolved it in a strong acid or alkali? If these questions have aroused your curiosity then continue reading for information on the many properties of silicon dioxide and how these properties make it a useful compound in more ways than one.
Silicon dioxide is found in nature in mainly two forms: crystalline and amorphous. Silica is also a constituent of gemstones and traces of the compound have also been found in volcanic rocks. It is the most abundant compound of silicon, found on the earth's crust and also one of the most commonly found oxides. Silica in pure sand is the amorphous form while quartz is an example of crystalline silica found in nature.
Molecular Structure of Silicon Dioxide
Silica occurs in nature in more than 13 different structures, each one slightly varying from the others. The physical properties might be different but the chemical properties remain the same, irrespective of the structure. In order to obtain a clearer understanding of the many properties of silicon dioxide or silica, it is important that we have the basic idea about the molecular structure of this oxide of silicon.
The building block of the structure of silica is the SiO4 unit. The structure of crystalline forms of silica is represented as continuous links of the SiO4 unit. The image above shows the structure of silicon dioxide. Silicon dioxide is formed when silicon is exposed to the oxygen in the atmosphere. If you look at the atomic model of silicon dioxide, you'll see that the four oxygen atoms (shown in blue) are located far apart from the silicon atom at the centre (shown in green), with the molecule forming a tetrahedral structure (as shown by the thin black lines). Here are some facts about the molecular structure of silicon dioxide.
* The angle formed between each -O-Si-O- bond is 109.5 degrees. However, in quartz, this angle is around 144 degrees.
* There is uneven charge distribution between parts of the tetrahedron, with the center being highly positive and the corners being strongly negative. This is because of the strong polarity of the Si-O bond.
* In gaseous state, the structure of silicon dioxide forms linear O=Si=O molecules.
* Each individual SiO4 tetrahedron is connected with adjacent tetrahedrons at the corners, forming a three-dimensional structure.
* The "bridge" formed by the oxygen atoms between the silicon atoms of adjacent tetrahedrons, is responsible for some of the unique properties of silicon dioxide.
* The tetrahedral shape is responsible for the rigidity of the silicon dioxide molecule.
* The length of the Si-O bond is 0.16 nm.
You might be wondering why the molecular formula of silicon dioxide is SiO2 when the structure shows four atoms of oxygen surrounding each silicon atom. Well, the reason is every individual tetrahedron shares each of its four oxygen atoms with the neighboring tetrahedron, which makes the net chemical formula to be SiO2. In addition to quartz, there are many other crystalline forms of silicon dioxide found in nature, under different temperature and pressure conditions. In high temperature conditions, silica is found in the form of tridymite and cristobalite whereas in regions of high pressure, it is found as seifertite and coesite.
Properties of Silicon Dioxide
Now that you have a clear understanding about the structure of silica, let's have a look at the physical and chemical properties of this compound.
Physical Properties of Silicon Dioxide
Due to the tetrahedral structure, the melting point of silicon dioxide is very high. The strong silicon oxygen covalent bonds get broken at very high temperatures, close to 1700oC. Also, silicon dioxide is very hard and rigid and this is again due to the strong covalent bond between silicon and oxygen. Due to the absence of free electrons within the molecular structure, silicon dioxide is a very bad conductor of electricity and acts as an insulator. Silicon dioxide is insoluble in water and in all organic solvents. However, it is soluble in alkalies and hydrofluoric acid. The table given below enlists the values for some of the physical properties of silicon dioxide, both crystalline and amorphous.
Physical Property Unit Crystalline Silica Amorphous Silica
Melting Point degree Celsius approx 1700 approx 1700
Density g cm-3 2.6 2.2
Refractive Index - 1.46 1.46
Resistivity ohm-cm 1012 - 1016 greater than 1018
Thermal Conductivity Wm-1K 1.3 1.4
Poisson's Ratio - 0.17 0.165
Coefficient of Thermal Expansion K-1 7.64 x 10-7 5.4 x 10-7
Chemical Properties of Silicon Dioxide
Silicon dioxide reacts with very few substances. Here we'll look into some of the common reactions of silicon dioxide and what are the products formed by each one of them.
Reacting Substance Reaction Conditions Main Product Formed Chemical Equation
Strong alkalis such as sodium hydroxide (NaOH) and potassium hydroxide (KOH) Crystalline silicon dioxide dissolves very slowly in hot alkaline solutions whereas its amorphous form reacts with alkalis at room temperature. Silicates of potassium or sodium SiO2 + 2 KOH → K2SiO3 + H2O
SiO2 + 2 NaOH → Na2SiO3 + H2O
Hydrofluoric acid (HF) Quartz does not react with other acids but dissolves in hydrofluoric acid and the reaction takes place at room temperature. Hydrofluorosilicic acid (H2SiF6) SiO2 + 6 HF → H2SiF6 + 2 H2O
Sodium Carbonate (Na2CO3) and potassium carbonate (K2CO3) Silicon dioxide reacts with molten carbonates of sodium and potassium. Silicates of sodium and potassium (K2SiO3 and Na2SiO3) SiO2 + K2CO3→ K2SiO3+ CO2
SiO2 + Na2CO3→ Na2SiO3+ CO2
Calcium carbonate (CaCO3) At very high temperatures above 600oC, quartz reacts with alkaline substances such as limestone or calcium carbonate. Calcium silicate (Ca3Si3O9), commonly known as wollastonite 3 SiO2 + 3 CaCO3 → Ca3Si3O9 + 3 CO2
Carbon Natural silica or quartz reacts with carbon at around 2000oC to give silicon. This reaction is used for obtaining silicon from its ore. Silicon (Si) SiO2 + 2 C → Si + 2 CO
Water Under high temperature and pressure conditions, silica is hydrolyzed by water to form hydroxide of silicon, which is highly unstable. Silicon hydroxide (Si(OH)4) SiO2 + 2H2O → Si(OH)4
Uses of Silicon Dioxide
One of the most common uses is silicon dioxide in food, where it is used as an additive. Following are some other important applications of silicon dioxide.
* Quartz is used in the glass industry as a raw material for manufacturing glass.
* Silica is used as a raw material for manufacturing concrete.
* Silica is added to varnishes because of its hardness and resistance to scratch.
* Amorphous silica is added as fillers to the rubber during the manufacturing of tires. This helps reduce the fuel consumption of the vehicle.
* Silica is used in the production of silicon.
* Due to the fact that silica is a good insulator, it is used as a filler material in electronic circuits.
* Because of its piezoelectric properties, quartz is used in transducers.
* It is a substance used for making optical fibers.
* Due to its ability to absorb moisture, silica is used as a desiccant.
Silicon dioxide is the most common oxide found on earth. Hope this article has helped you get a basic understanding of the properties of silicon dioxide and also what makes this oxide unique in more ways than one.
_________________
mrsamct |
|

















